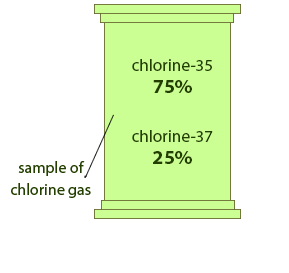
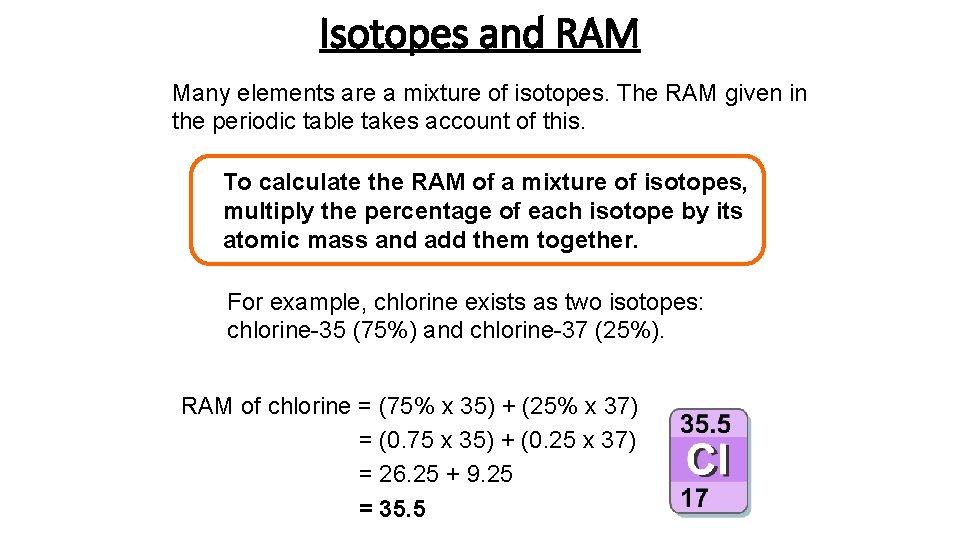
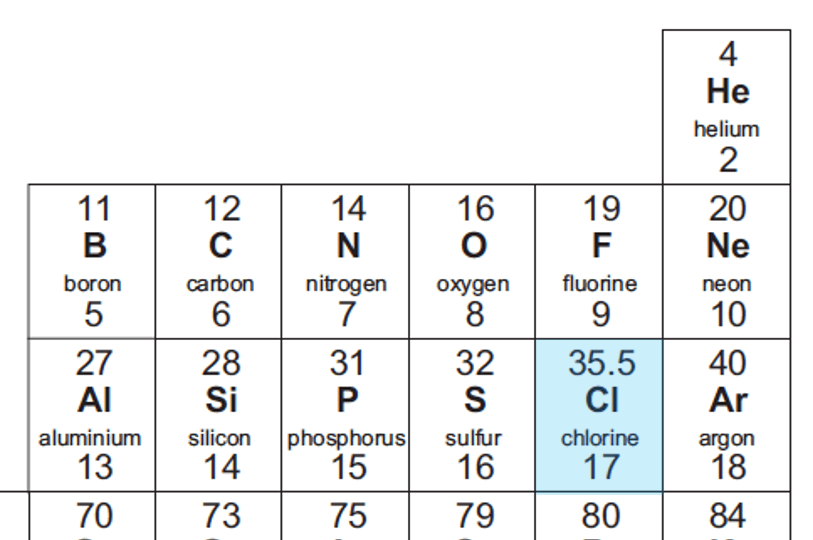
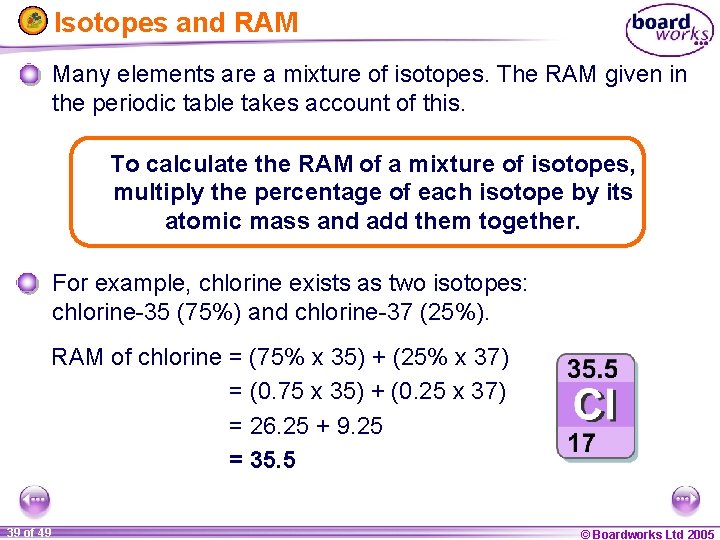
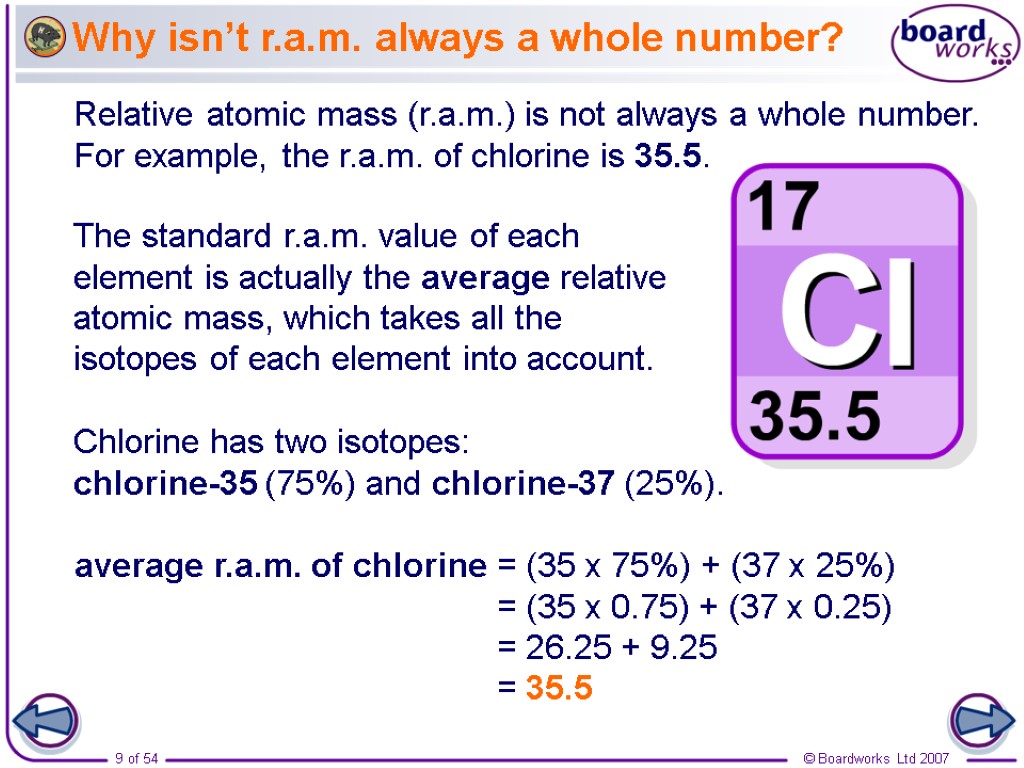
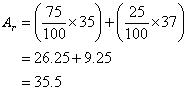Relative Atomic Mass Mass of atoms is incredibly small! So we have to use relative atomic mass. The symbol for relative atomic mass is Ar Carbon- 12 atom. - ppt download

Relative atomic mass Atoms are too small to be weighed individually, even with the best balance! We can, however, compare the masses of different atoms. - ppt download

Defining how to calculate relative atomic mass of element relative isotopic mass definition gcse chemistry Calculations igcse O Level revision notes

Chlorine And Ph Meter Drinking Water Ram Chipset Digital Ph Meter For Aquarium - Buy Ph Meter Accessories,Ph Meter Water Proof,Smart Sensor Ph Meter Product on Alibaba.com