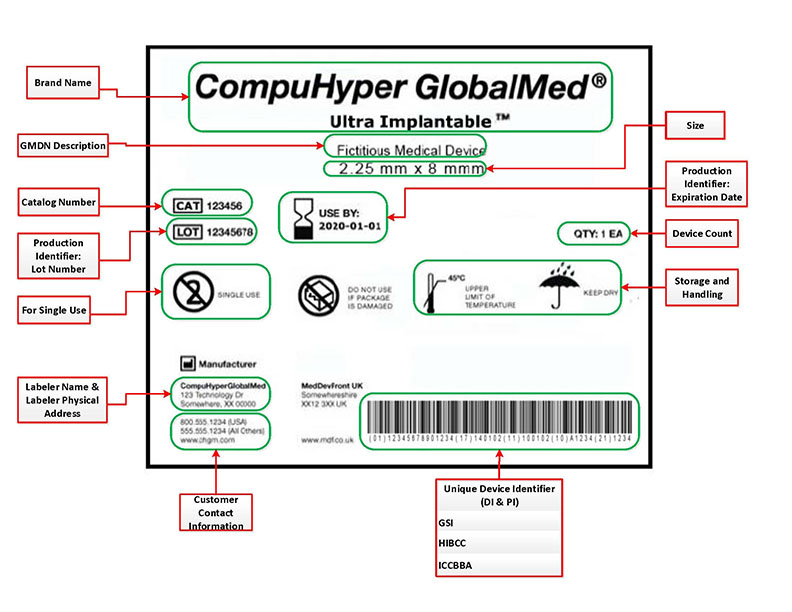
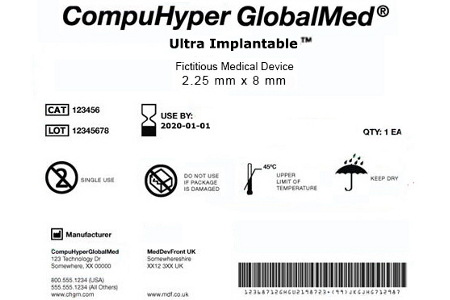
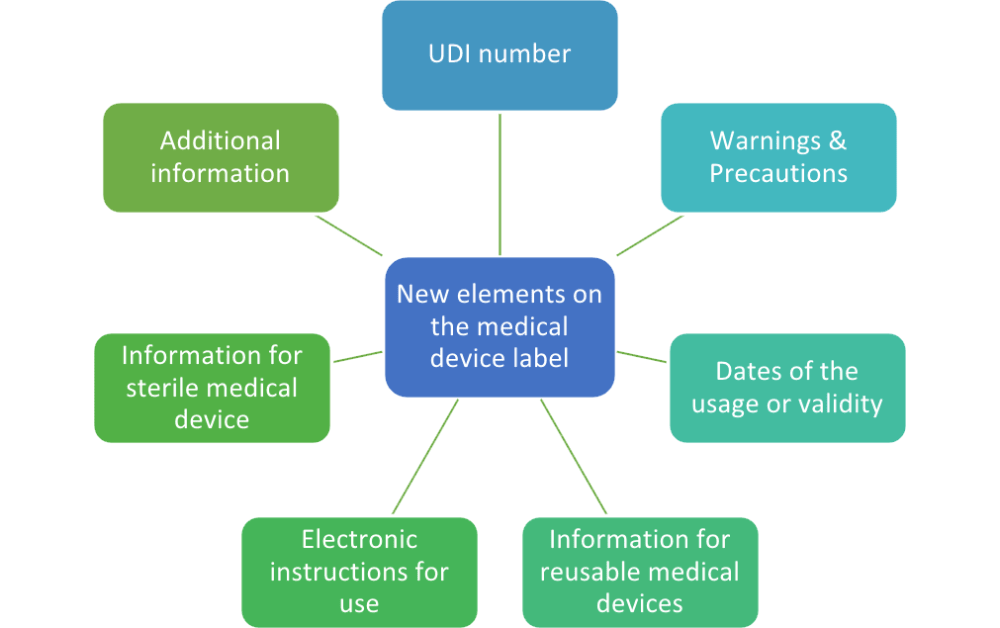
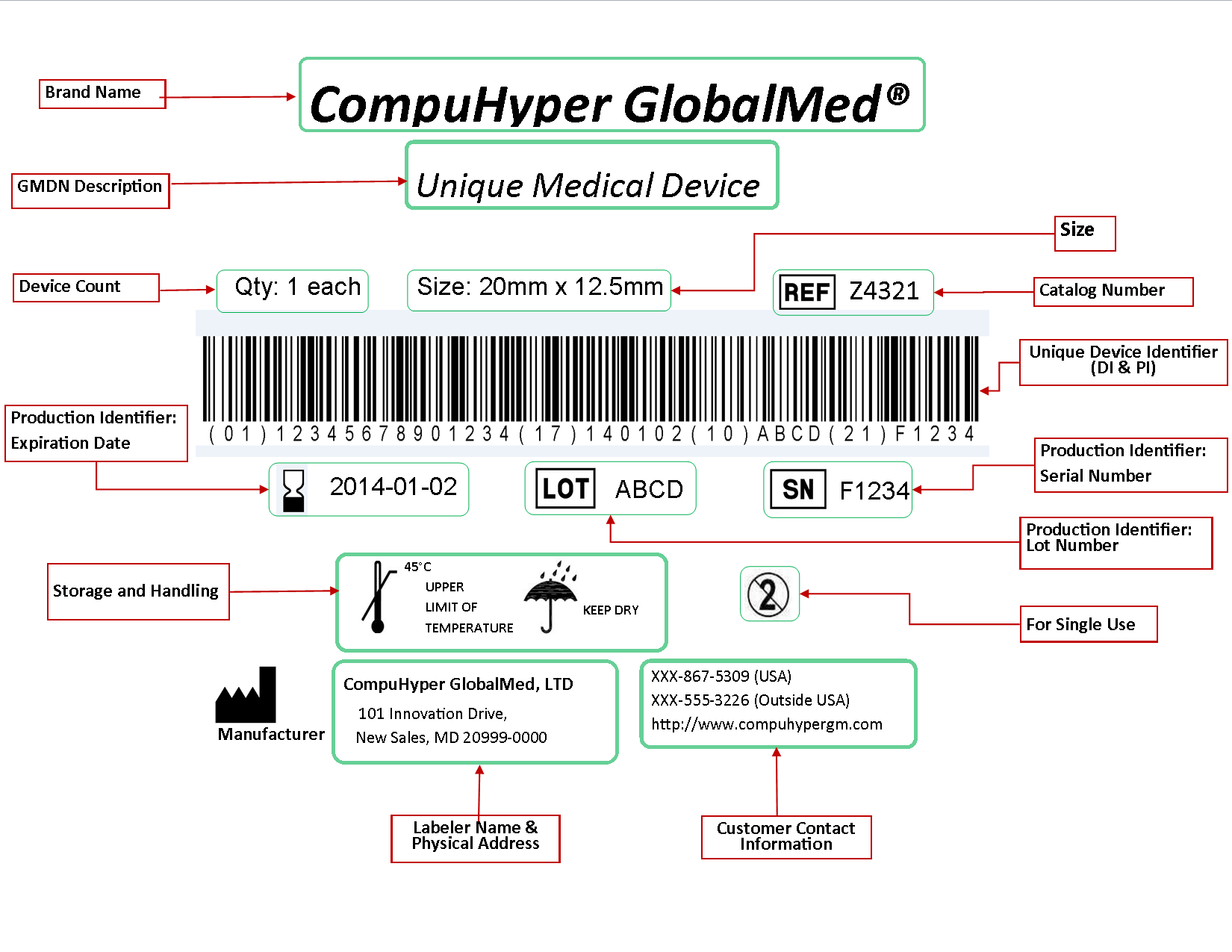
Health Canada's Action Plan on Medical Devices: Continuously Improving Safety, Effectiveness and Quality - Canada.ca
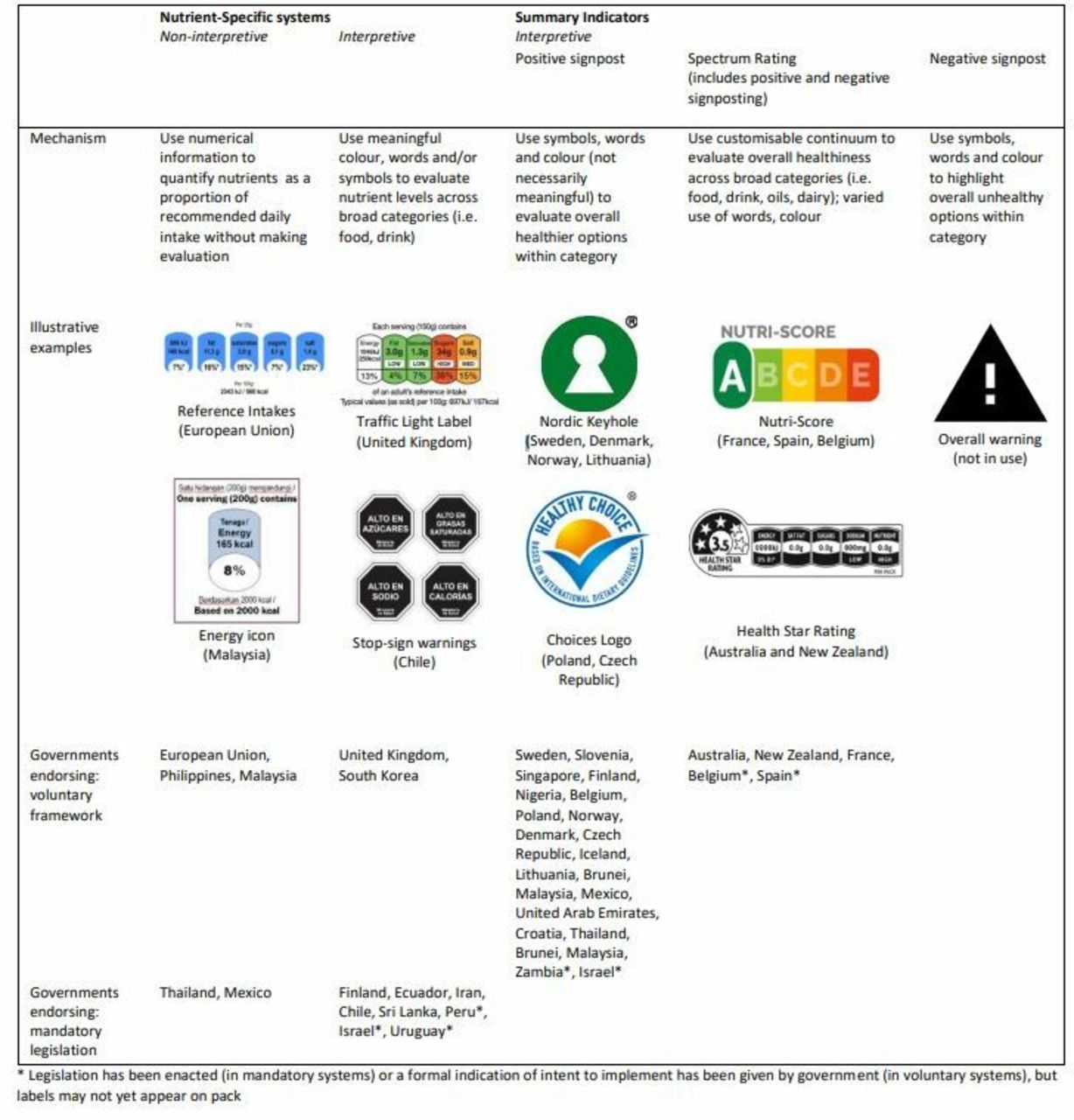
Front-of-pack nutrition labelling to promote healthier diets: current practice and opportunities to strengthen regulation worldwide | BMJ Global Health

Design History File (DHF) vs. Device Master Record (DMR) vs. Device History Record (DHR): What's the Difference?