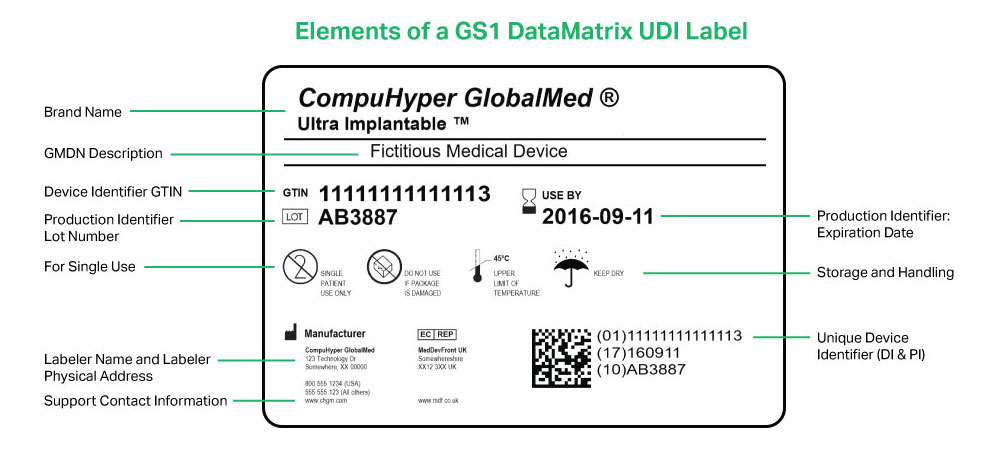
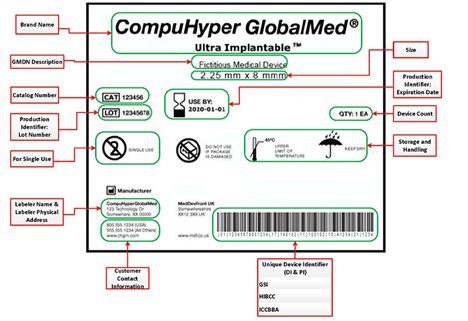
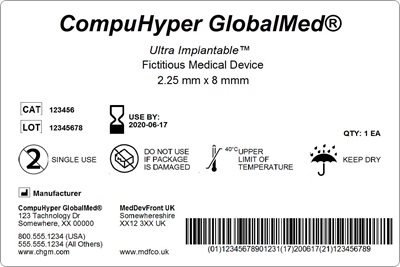
PRISYM ID Twitterren: "PRISYM 360 provides approval and change workflow tools to ensure control over label content and compliance with the myriad regulations for #medicaldevice manufacturers and #pharma. Learn more: https://t.co/Vcpjbuqut7 https://t.co ...

No more paper medical instructions - dokspot: helping MedTech companies go paperless — OneMillionSparks

Unique device identification and traceability for medical software: A major challenge for manufacturers in an ever-evolving marketplace - ScienceDirect